Medical Devices
SZUTEST Konformitätsbewertungsstelle GmbH is recognized by the Central Authority of the Länder for Health Protection with regard to Medicinal Products and Medical Devices (ZLG) and EU Commission, as a Notified Body with the identification number 2975 under the Medical Device Regulation (EU) 2017/745
WHAT SERVICES DOES SZUTEST OFFER FOR MEDICAL DEVICES?
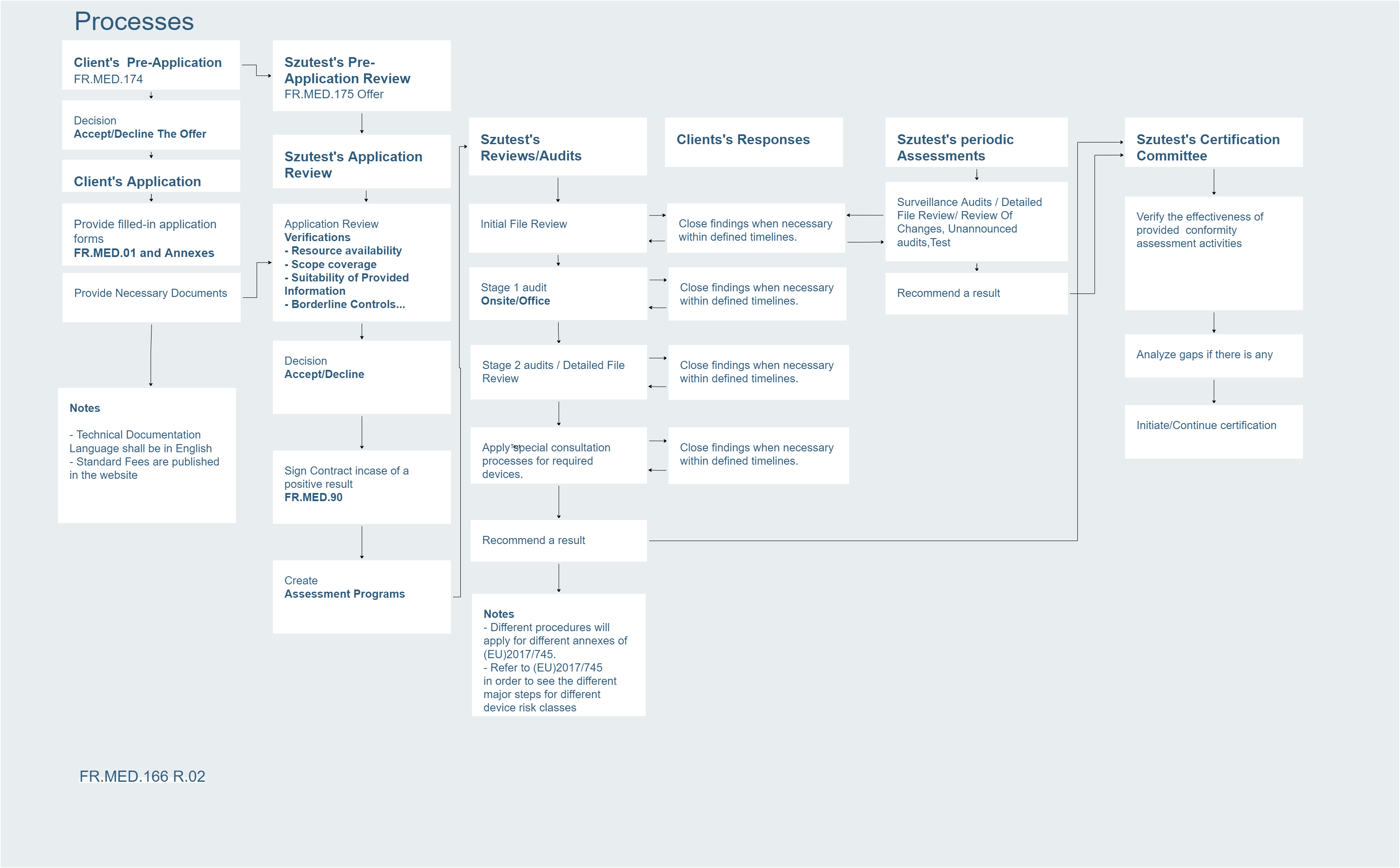
The scope of the application includes MDR Medical Device Regulation (EU) 2017/745 in Annex IX Part I, Annex IX Part II and Annex XI Part A.
Medical Devices
SZUTEST Konformitätsbewertungsstelle GmbH is recognized by the Central Authority of the Länder for Health Protection with regard to Medicinal Products and Medical Devices (ZLG) and EU Commission, as a Notified Body with the identification number 2975 under the Medical Device Regulation (EU) 2017/745
WHAT SERVICES DOES SZUTEST OFFER FOR MEDICAL DEVICES?
The scope of the application includes MDR Medical Device Regulation (EU) 2017/745 in Annex IX Part I, Annex IX Part II and Annex XI Part A.


